

Montego Bay-based pharmaceutical company, Indies Pharma Jamaica Limited has been selected as the preferred partner for the private sector distribution of the Indian manufactured COVID-19 vaccine, COVAXIN in Jamaica.
The company was selected as the preferred partner by its parent company, Bioprist Pharmaceuticals, which is also based in the second city and markets pharmaceuticals in the wider Caribbean encompassing the Cayman Islands, Bahamas, Turks & Caicos Islands, Haiti, Dominican Republic, Eastern Caribbean Islands, Trinidad & Tobago and Guyana.
On April 1 this year, the board of Indies Pharma Jamaica, which is listed on the Jamaica Stock Exchange (JSE), agreed to move forward with the distribution deal for COVAXIN in Jamaica. This, is however subject to approval from the Government of Jamaica.
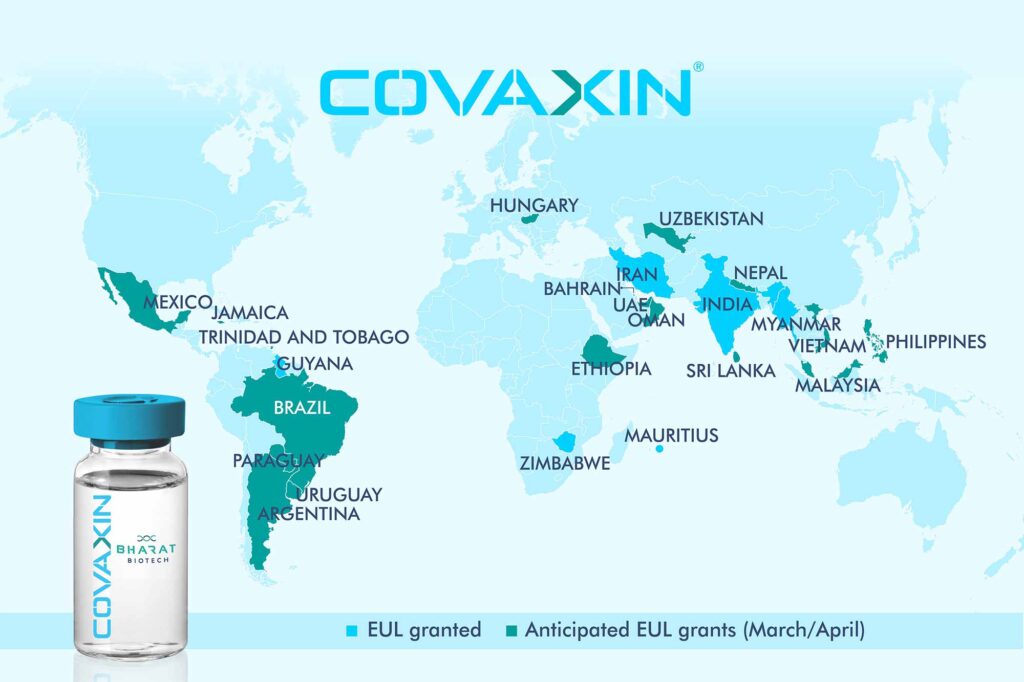
COVAXIN will be supplied from Bharat Biotech, a company which is duly registered in India. Indies Pharma Corporate Secretary has disclosed to the JSE the company’s intent to distribute the said vaccine.
Withholding permission for COVAXIN use in Jamaica
Last month Bioprist Pharmaceuticals, which is led by medical doctor and entrepreneur, Dr Guna Muppuri signed a deal for the exclusive importation and distribution in Jamaica of COVAXIN.
The vaccine is yet to be approved for emergency use authorisation by the World Health Organization (WHO), which has caused the Jamaican authorities to withhold permission for the use of the product in the island.
The management of Bioprist and Bharat Biotech are now in talks with the Jamaican Government and state pharmaceutical procurement company, the National Health Fund, as they seek to win over approval of the vaccine in Jamaica.
The Government has so far held fast to its position of maintaining a rigorous regime for COVID-19 drug approval, involving inclusion in the WHO emergency use listing or authorisation by the US Food and Drug Administration (FDA), Health Canada, or the European Medicines Agency (EMA).
Officials of Bioprist and Bharat Biotech have expressed confidence that Covaxin will receive WHO approval once the global health body has reviewed data from the drug’s recently concluded phase-three trial involving nearly 26,000 people and showing an efficacy rate of 81 per cent and kits suitability for persons between the ages of 12 and 85 years.
Dr Muppuri advised that the Indian manufacturers have given Jamaica a commitment to deliver 100,000 doses of the life-saving Vcovid-19 vaccine by this month.






Comments