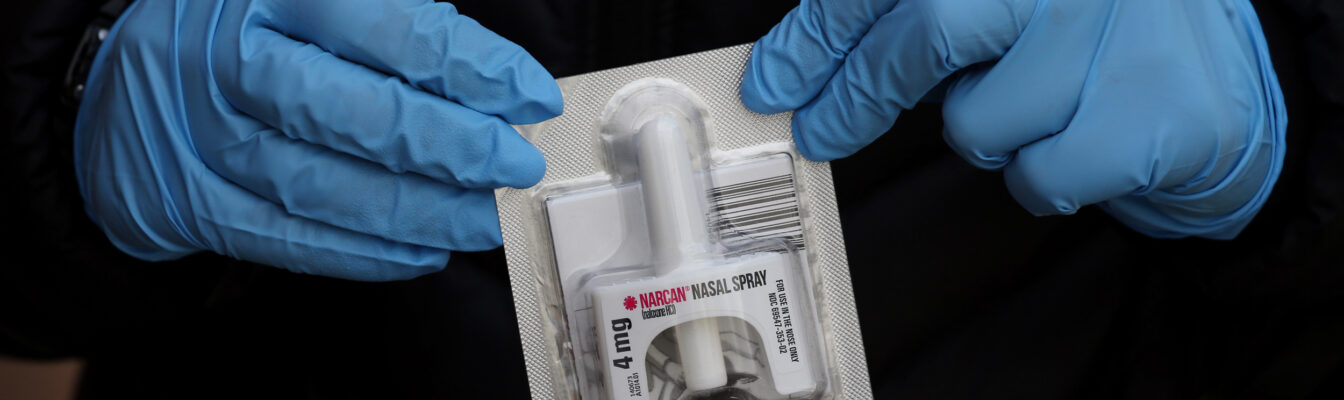
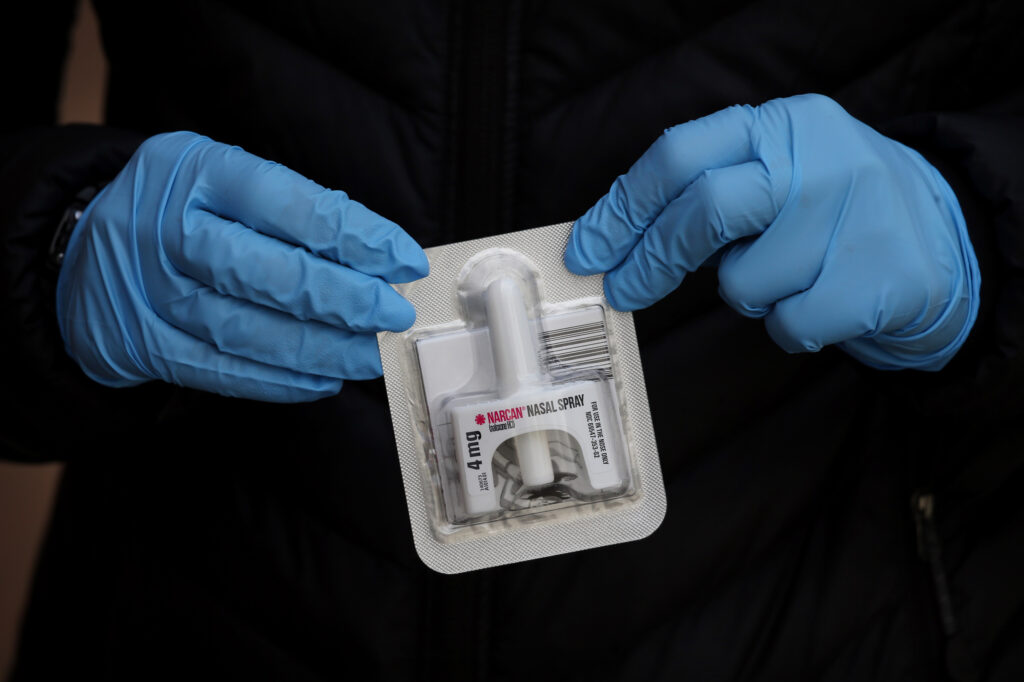
The first over-the-counter nasal spray to reverse the effects of opioid overdose was approved on Wednesday (March 29) by the U.S. Food and Drug Administration.
Narcan, 4 milligram (mg) naloxone hydrochloride nasal spray is the first naloxone product approved for use without a prescription.
The FDA in a media statement said: “Today’s action paves the way for the life-saving medication to reverse an opioid overdose to be sold directly to consumers in places like drug stores, convenience stores, grocery stores and gas stations, as well as online.”
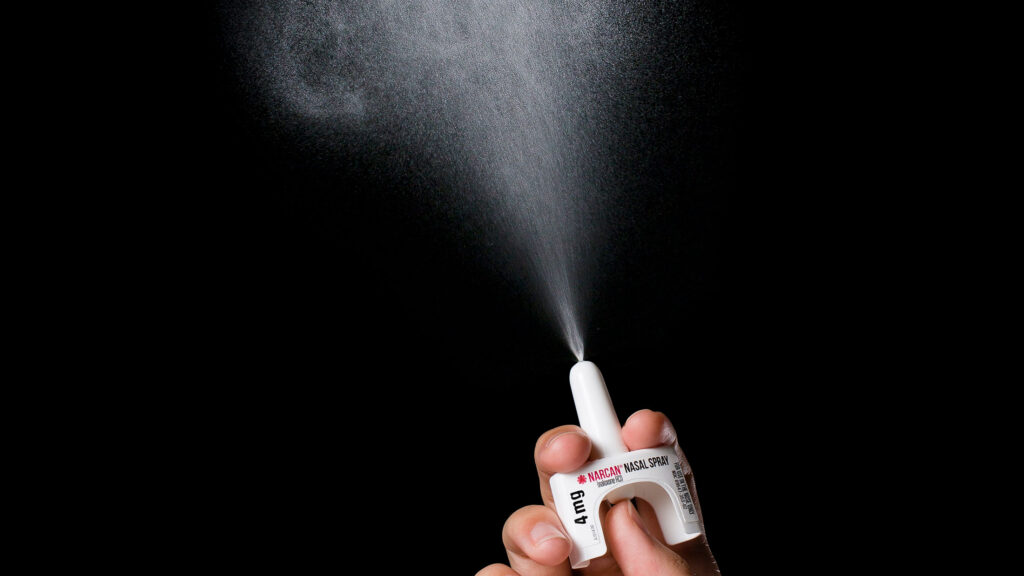
The FDA says the timeline for availability and the cost of the over-the-counter drug will be determined by the manufacturer, Opiant Pharmaceuticals.
Naloxone is sold under the brand name Narcan after it was first approved by FDA in 2015 to be used as a prescription drug.
The FDA said it will continue to work with all stakeholders to facilitate the continued availability of naloxone nasal spray products during the time needed to implement the transition to over-the-counter status, which may take months.
FDA’s recent approval of the product is aimed at tackling the current crisis of drug overdose in the U.S.






Comments